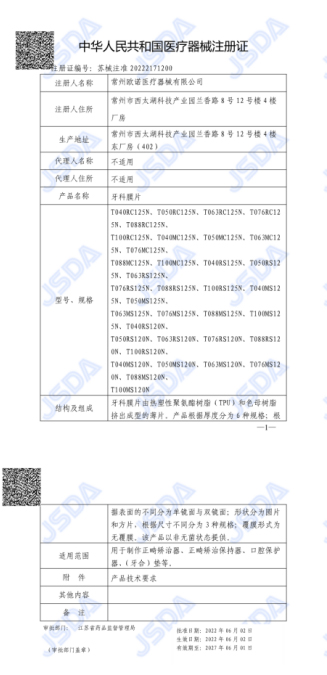
After the review of enterprise technical requirements, multiple device inspections, hundreds of system assessments and on-site reviews, Changzhou Ono Medical Devices Co., Ltd., a holding joint venture of Shanghai prismlab polyson group, officially obtained the registration certificate of dental diaphragm medical devices issued by Jiangsu food and Drug Administration! This shows that the invisible orthodontic diaphragms developed and produced by prinson fully meet the market standards, marking that prinson has further improved the construction of various systems and standards, and has taken a solid step towards digital dentistry! The construction of departments and standards has taken a solid step towards digital dentistry!
Post time: Jul-09-2022

